Society of Toxicology (SOT)
2025-01-22
Society of Toxicology (SOT)
Workshop
March 16-20, 2025
Orlando, FL
| Session Presentation
Session Title: Advanced Capabilities for Ocular Drug Development Using NHP Models
Date & Time: March 17th (Mon), 1:45-2:45pm
Location: West Concourse of the Orange County Convention Center,Room W208B
Presenting Author:
Wankun Xie, MD; PhD
Director of Ophthalmology
Wankun Xie serves as Director of Ophthalmology at JOINN laboratories. He is responsible to build and develop ocular service capabilities to provide partners and clients with novel and better solutions for the needs in ocular drug research.
Before joining JOINN, Wankun was the research scientist in ophthalmology at Texas A&M University. Over the last 15 years, he has been involved in various international projects of translational ocular research, and lead the team to successfully complete hundreds of ocular studies and secure dozens of the IND approvals globally.
Wankun received doctoral degree in ophthalmology from Peking University in China. Following his studies, he practiced as an ophthalmologist for several years and then received clinical training in the US before moving into industry roles.
| AACT Career Workshop
Date & Time: March 17th (Mon), 5:00-6:00 pm
Location: Windermere Ballroom X, Hyatt Regency
Presenting Author:
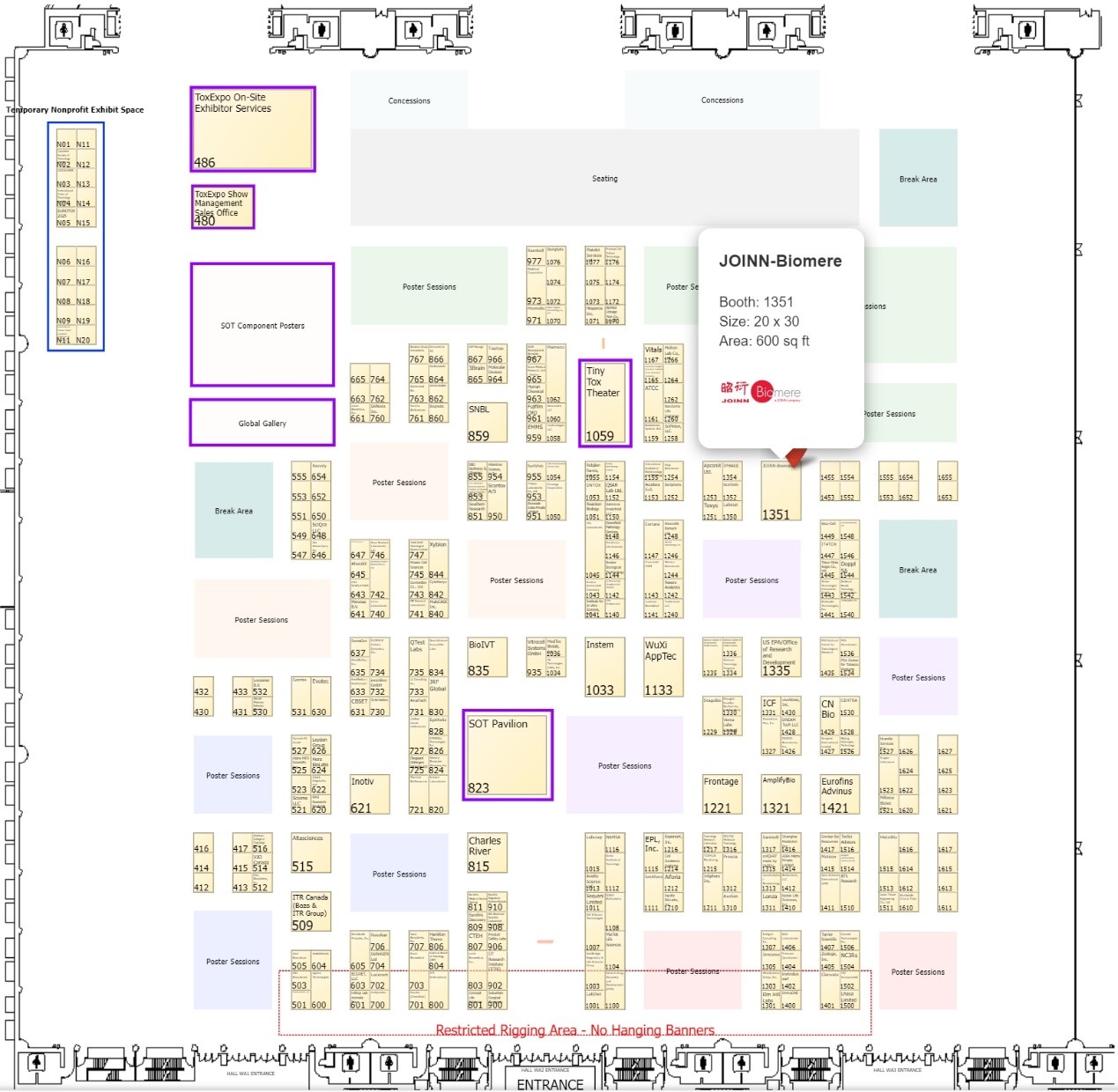
| Poster (Free posters can be obtained at booth 1351)
01
Abstract Number/Poster Board number: 4254/Q770
Abstract Title: Analysis of Toxicity Characteristics of GLP-1 Receptor Agonists in Non-clinical Safety Evaluation
Introduction:
Glucagon-like peptide-1 receptor agonist (GLP-1RA) is a new hypoglycemic drug class that can reduce blood glucose and help with weight loss by activating the GLP-1 receptor. GLP-1RA also has multiple clinical benefits, including controlling body weight and improving non-alcoholic fatty liver disease, and is gradually becoming the primary prescription drug for the treatment of diabetes. This abstract summarizes the results of the toxicity studies of GLP-1RA mainly injections conducted in our facility, combined with the toxicity characteristics of similar marketed products, to provide a reference basis for non-clinical safety evaluation and toxicity analysis of such products.
Date & Time: March 18th (Mon), 1:45-4:15 pm
Session Title: Safety Assessment:Non-pharmaceutical
Presenting Author: Jinling Ma
02
Abstract Number/Poster Board number: 4166/N676
Abstract Title: In vivo Imaging and Quantification of Retinal and Choroidal Vasculature in Cynomolgus Monkeys using Optical Coherence Tomography Angiography
Introduction:
Optical coherence tomography angiography (OCTA) is a novel non-invasive technique for visualizing the retinal and choroidal microvascular system. Non-human primates (NHP) are widely used in preclinical ocular research due to their anatomical and genetic similarities shared with human eyes. The purpose of this study was to characterize the OCTA feature of NHP, and establish standardized methods for evaluating retinal vascular complexes in NHP for preclinical ocular research.
Date & Time: March 18th (Mon), 1:45-4:15 pm
Session Title: Ocular Toxicology
Presenting Author: Wankun Xie
03
Abstract Number/Poster Board number: 3670/A130
Abstract Title: Pharmacodynamic Effect and Toxicity Study of Multi-specific Antibodies in a Humanized Mouse Model with Tumor-Bearing
Introduction:
T-cell engagers (bispecific antibodies) can bind to both tumor cells and immune cells such as T-cells, and direct immune cells to kill tumor cells by the dual binding. However, the bispecific antibodies can be easily getting into a status being depleted which cause activated T-cells becoming unresponsive shortly after application due to lack of costimulatory molecules. Based the above observation, tri-specific antibody with adding a costimulatory molecule was created to avoid incapacity of T-cells activated by a single signal which expects to improve T-cell killing efficacy to tumor cells. This study evaluated the anti-cancer efficacy and potential toxicity of CD3/CD20/CD28 antibodies and Blinatumomab analog monoclonal antibodies on Raji tumor-bearing NPG mouse model with human hematopoietic stem cells (HSC) reconstitution, as well as their in vitro effect on human peripheral blood mononuclear cells (PBMCs) activation.
Date & Time: March 18th (Mon), 1:45-4:15 pm
Session Title: Immunotoxicity I
Presenting Author: Li Sun
04
Abstract Number/Poster Board number: 3644/A102
Abstract Title: Comparison of Cytokine Release Syndrome induced by Chimeric Antigen Receptor T Cells in Humanized NOG-EXL mice and NOG mice
Introduction:
T cell-mediated cancer immunotherapies, including chimeric antigen receptor (CAR) T cell-therapy and bispecific antibody that recruits cytotoxic T lymphocytes (CTLs) to cancer cells, directly recognize surface antigens of cancer cells regardless of MHC restriction. Despite the remarkable efficacy against malignancies in clinical application, the CAR T cell-therapy is frequently accompanied with severe cytokine release syndrome (CRS), which is one of the draw backs of CAR T cell-immunotherapy. We previously reported activation of infused CAR T cells and regression of tumor burden in tumor bearing NOG mice, in which the human cytokine release in serum was subtle. It is considered that a variety of human immune cells contribute to the CRS, so we evaluated the CRS in the NOG-EXL mice with human hematopoietic stem cell (HSC) transplantation and tumor engraftment. In the humanized NOG-EXL model, we found expansions of human T, B and myeloid cells, as well as elevated human cytokine levels after CAR-T cell injection, in mouse peripheral blood.
Date & Time: March 18th (Mon), 1:45-4:15 pm
Session Title: Immunotoxicity I
Presenting Author: Yang Cao
05
Abstract Number/Poster Board number: 3641/A101
Abstract Title: Therapeutic effects of anti-TNF-α monoclonal antibody on cytokine release induced by CAR-T cells targeting CD22
Introduction:
Although cytokine release syndrome (CRS) is the most common clinical side effect of CAR-T cell therapies, it is rarely seen in preclinical studies using tumor-bearing model of immune deficient mice. When we evaluated the efficacy and safety of a CD22-specific CAR-T cell product in a NOG-Raji mouse model, CAR-T cells associated toxic reactions were found, including transient weight loss and animal death. In subsequent data analyses, abnormally elevated levels of TNF-α were considered to be associated with such toxic reactions in mice. Treatment with anti-TNF-α monoclonal antibody (Adalimumab) antagonized weight loss and reduced mortality rate in mice without affecting the antitumor effects of CAR-T cells. Our results suggest that elevated peripheral blood TNF-α level may be a future clinical toxicity marker for this CAR-T cell product, and TNF-α neutralization therapies maybe the feasible scheme to prevent and treat potential clinical CRS.
Date & Time: March 18th (Mon), 1:45-4:15 pm
Session Title: Immunotoxicity I
Presenting Author: Yang Chen
06
Abstract Number/Poster Board number: 3668-A128
Abstract Title: Immunogenicity Concerns in Preclinical Safety Evaluation of Biologics in Non-human Primates
Introduction:
Biological drugs such as monoclonal antibodies (mAbs), bispecific antibodies, and fusion proteins directed towards soluble biological targets make a great improvement in medical therapy of difficult to treat or rare diseases. Non-human Primates (NHPs) have been demonstrated to be a valuable animal species for preclinical safety evaluation of biologics, as they have good predictive value for pharmacodynamics, toxicological impacts, and physiological effects, However, immunogenicity is still a big concern for safety evaluation of biologics conducted in NHPs that can may compromise the predictive value for human adverse effects. This study aims to present specific parameters or methods for immunogenicity recognition and intervention regime in preclinical safety evaluation studies of biological products.
Date & Time: March 18th (Mon), 1:45-4:15 pm
Session Title: Immunotoxicity I
Presenting Author: Yongbin Zhang
07
Abstract Number/Poster Board number: 3612-P767
Abstract Title: Immunogenicity Concerns in Preclinical Safety Evaluation of Biologics in Sprague-Dawley Rats
Introduction:
The development of biologics such as monoclonal antibodies (mAbs), bispecific antibodies, fusion proteins etc., have recently contributed significantly to the improvement of medical treatment of various diseases relating to cardiovascular system, respiratory system, cancer, hematopoietic disease, autoimmune disease etc. Animal studies with the Sprague-Dawley (SD) rat is a common and valuable tool for safety evaluation of the biologics. However, immunogenicity is still a concern and/or challenge when a safety evaluation study for biologics is conducted in rats which may compromise the predictive value for human adverse effects. However, rats do have good predictive value for human pharmacodynamics, and toxicological impacts. The purpose of this review was to assess the preclinical data of biologics toxicity studies to investigate immunogenicity of biological products.
Date & time: March 17th (Mon), 9:15-11:45 am
Session Title: Immunotoxicity I
Presenting Author: Luke Zhang














